Collaboration with Dr. Suleiman Al Habib Hospital for continuous medical education
Tanyx® obtains MOHAP registration approval
Goro Healthcare is proud to announce that Tanyx® – an innovative new concept in pain management – has been granted registration approval by the Ministry of Health And Prevention in the United Arab Emirates and launched in the country.
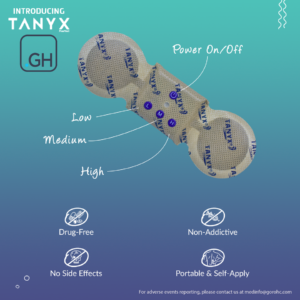
TANYX® is the world’s first US-FDA approved, self-applicable and portable TENS (Transcutaneous Electrical Nerve Stimulation) technology-based pain relief device with patents registered in over 40 countries to date.
Tanyx® is smaller than any other TENS device on the market and is specifically calibrated for pain relief. The device is wireless, portable, and can be self-applied without any assistance from healthcare professionals, allowing patients to take greater control of their pain treatment and recovery. TENS technology uses controlled electrical stimulation, that when in contact with the skin, blocks pain signals going to the CNS (Central Nervous System).
The launch of Tanyx®, underlines our commitment to deliver unique and patient-centric healthcare innovations to our communities in the region. Tanyx® is available for order immediately from our website and on our Amazon store.
For more information, please visit:
Our Product:
Order on Amazon:
Recent News
Launching the Demophorius portfolio in UAE and Qatar
